Water Purification :
 Many floculants need alkali as an assistant floculant. If additional alkali is not added, the total alkalinity is reduced and part of aluminium sulphate, being water soluble may pass through the filter. As aluminium hydroxide and metals precipitate downwards, clear water arrives at the top and is decanted.
Many floculants need alkali as an assistant floculant. If additional alkali is not added, the total alkalinity is reduced and part of aluminium sulphate, being water soluble may pass through the filter. As aluminium hydroxide and metals precipitate downwards, clear water arrives at the top and is decanted.
Lime-soda process
In this process lime and sodium carbonate are added to precipitate the calcium and magnesium salts as temporary hardness.
 For permanent hardness the reactions are
For permanent hardness the reactions are

 The softened water is usually slightly alkaline with 1-4 degree residual hardness.
The softened water is usually slightly alkaline with 1-4 degree residual hardness.
Base exchange process
When hard water is passed through a bed consisting of zeolites, which are synthetic material systems composed of complex sodium, aluminium and silicate salts (Na2Z), the calcium and magnesium ions are exchanged. The displacing reactions for temporary hardness are :
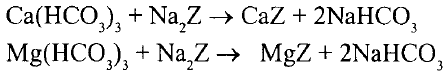 For permanent hardness the reactions are :
For permanent hardness the reactions are :
 The process is reversible and the bed (Na2Z) can be regenerated by passing concentrated salt solution. This method of water softening yields a very soft water (0.5-1 o hardness).
The process is reversible and the bed (Na2Z) can be regenerated by passing concentrated salt solution. This method of water softening yields a very soft water (0.5-1 o hardness).
 Water softening by demineralisation systems can be accomplished using either mixed bed (both cation and anion resin in one bed) or two bed system (resins remain seperated according to their charges). The active sites of the resins are limited and the sites are filled when water passes through these columns and must be regenerated again. Their ion-exchange capacity is greater than that of zeolite.
Water softening by demineralisation systems can be accomplished using either mixed bed (both cation and anion resin in one bed) or two bed system (resins remain seperated according to their charges). The active sites of the resins are limited and the sites are filled when water passes through these columns and must be regenerated again. Their ion-exchange capacity is greater than that of zeolite.
Conventional softening treatment plant may not remove the impurities in water to the recommended permissible level. Demineralized or reverse osmosis technique is needed for removal of TDS from water but is costly. Water purification in the process house normally consists of flocculation, sedimentation, filtration and ion exchange. Hard water is normally softened using one or combination of methods.
Soda-alum process
Hard water is first pumped into the reaction tank and then aluminium sulphate is added to it as floculant. About 20-30 min is allowed to react and then the impurities are allowed to settle for about 30 min before filtration.
Soda-alum process
Hard water is first pumped into the reaction tank and then aluminium sulphate is added to it as floculant. About 20-30 min is allowed to react and then the impurities are allowed to settle for about 30 min before filtration.
Lime-soda process
In this process lime and sodium carbonate are added to precipitate the calcium and magnesium salts as temporary hardness.

Base exchange process
When hard water is passed through a bed consisting of zeolites, which are synthetic material systems composed of complex sodium, aluminium and silicate salts (Na2Z), the calcium and magnesium ions are exchanged. The displacing reactions for temporary hardness are :
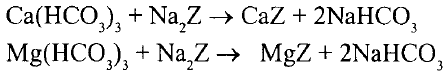









{ 0 comments... read them below or add one }
Post a Comment